i3- lewis structure molecular geometry|I3 : Bacolod I3- is an interesting and difficult molecule to deal with when it comes to chemical bonding. Although the molecular geometry is linear as . Tingnan ang higit pa Wildkard est une plateforme qui contient des produits sains en un seul endroit.La vente en ligne des chaussures et de vêtements de sport en Tunisie.Des survê.
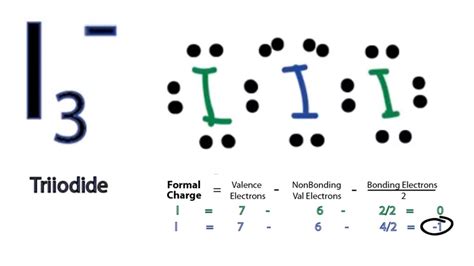
i3- lewis structure molecular geometry,If we take another look at the Lewis Structure of the ion I3-, we can easily see that the central iodine has three lone pairs. The lone pairs are positioned equatorially due to repulsion. Hence, the other two iodine atoms are placed axially and the bond angle made is 1800. If we have focused on the formal . Tingnan ang higit pa
Anyone wanting to know in-depth about a molecule needs to learn about the Lewis Structure. Why is it so necessary to have an idea on . Tingnan ang higit paThe hybridization of I3 (Triiodide ion) is sp3d. The way we draw the structure of molecular compounds on paper is just a two . Tingnan ang higit pa
Triiodide ion is a shining example of 3-center-4-electron chemical bonding. Now, what does it signify? Before that, a brief summary of MO or Molecular Orbital theory is . Tingnan ang higit paI3- is an interesting and difficult molecule to deal with when it comes to chemical bonding. Although the molecular geometry is linear as . Tingnan ang higit pa The number of lone pairs in this molecule is 3, and the number of atoms sharing valence electrons is 2. Hence, 3+2=5 which .
I3- Lewis Structure - Triiodide Ion. This chemistry video tutorial explains how to draw the lewis structure of I3-. It also discusses the molecular geometry, bond angle, hybridization,. An explanation of the molecular geometry for the I3 - ion (Triiodide Ion) including a description of the I3 - bond angles. The electron geometry for the Trii. I 3 – Lewis structure shape: I 3 – lewis structure has a trigonal bipyramidal geometry which is well justified as the central atom contains 3 lone pairs of . 984. 191K views 10 years ago. A step-by-step explanation of how to draw the I3 - Lewis Dot Structure (Triiodide Ion). For the I3 - structure use the periodic table to find the total.For the I3- Lewis structure we first count the valence electrons for the I3- molecule using the periodic table. Once we know how many valence electrons there are in I3- we can .In I3-, each iodine atom has two lone pairs, giving us the following Lewis structure: This Lewis structure shows us that the triiodide ion has 22 valence electrons, a linear . I 3– Lewis structure. I 3– (triiodide) has three iodine atoms. In the I 3– Lewis structure, there are two single bonds around the iodine atom, with two other .
Learn how to draw the Lewis dot structure of [I3]-, a triiodide anion composed of three iodine atoms. Find out its molecular geometry, electron geometry, bond angle, hybridization, formal charges, and .
The first step in drawing a Lewis structure is to determine the total number of valence electrons in the molecule. For I3-, we add the valence electrons of each iodine atom and subtract one electron for the negative charge of the ion. This gives us: Number of valence electrons = 7 (I) x 3 + 1 (charge) = 22. 2. Draw the Lewis electron structure of the molecule or polyatomic ion. Determine the electron group arrangement around the central atom that minimizes repulsions. Assign an AX m E n .3. -. ) - Lewis Structure. In the lewis structure of triiodide ion (I 3- ), there are two I-I bonds. and one iodine atom is located as the center atom. Each iodine atom has 3 lone pairs and center iodine atom have -1 charge. We will learn how to draw the lewis structure of I 3- step by step in this tutorial.

Draw the Lewis structure for ICl3 and determine its electron pair geometry, molecular geometry, and hybridization of iodine. For PO_4^3-, phosphate ion, draw the Lewis structure (by counting valence electrons of each atom), determine the electron-domain geometry, molecular geometry, hybridization, and show the angle between the bonds .
I3 Lewis Structure Molecular Geometry Hybridization Polarity And Mo Diagram. I2 + I- —-> I3- This is the exergonic equilibrium leading to the formation of the ion where a positive flow of energy happens from the system to the surroundings. One of the major uses of this ion is due to its non-reactive property with starch which results in an .i3- lewis structure molecular geometryWrite the lewis structure and describe the molecular geometry at each carbon atom in the below compound: 2-Pentyne Draw the Lewis structure for each of the following ions or molecules. For each give (i) the molecular shape, (ii) the electron pair geometry at the central atom, and (iii) the hybridization of the central atom.I3 The molecular geometry of any molecule depends on its Lewis structure, the arrangement of atoms, and its electrons. In an H2O molecule, the Oxygen atom forms two single sigma bonds with Hydrogen atoms. Although these two Hydrogen atoms are arranged symmetrically in the plane, the two lone pairs of electrons on the Oxygen atom .i3- lewis structure molecular geometry I3 The molecular geometry of any molecule depends on its Lewis structure, the arrangement of atoms, and its electrons. In an H2O molecule, the Oxygen atom forms two single sigma bonds with Hydrogen atoms. Although these two Hydrogen atoms are arranged symmetrically in the plane, the two lone pairs of electrons on the Oxygen atom . Hello Guys,We are back with one of the most requested videos on Geometry of Molecules- I3- Lewis structure. It is a chemical formula for the Triiodide ion. T.
These pair of electrons present between the Iodine (I) atoms form a chemical bond, which bonds all the iodine atoms with each other in a I3- ion. Step #4: Complete the octet (or duplet) on outside atoms. If the valence electrons are left, then put the valence electrons pair on the central atom.

The total valence electron available for the NI3 lewis dot structure is 26. The hybridization of NI3 is Sp³. Nitrogen triiodide is slightly polar in nature. The molecular geometry of NI3 is trigonal pyramidal and its electron geometry is tetrahedral. Lewis structure of NI3 contains 1 lone pair and 3 bonded pairs.Figure 8.6.1 8.6. 1 shows the various molecular geometries for the five VESPR electronic geometries with 2 to 6 electron domains. When there are no lone pairs the molecular geometry is the electron (VESPR) geometry. When there are lone pairs, you need to look at the structure and recognize the names and bond angles. A step-by-step explanation of how to draw the I3 - Lewis Dot Structure (Triiodide Ion).For the I3 - structure use the periodic table to find the total number.
I3- Estructura de Lewis, Geometría, Hibridación: 7 pasos (resuelto) El ion triyoduro (I3⁻) consta de una disposición lineal de tres átomos de yodo (I), con el átomo I central unido a dos átomos I terminales. Tiene 7 electrones de valencia por átomo, más un electrón adicional debido a la carga negativa, totalizando 22 electrones.Triiodide is a model system in photochemistry. Its reaction mechanism has been studied in gas phase, solution and the solid state. In gas phase, the reaction proceeds in multiple pathways that include iodine molecule, metastable ions and iodine radicals as photoproducts, which are formed by two-body and three-body dissociation.
I quickly take you through how to draw the Lewis Structure of I3- (TriIodide Ion). I also go over hybridization, shape and bond angle.Asked for: molecular geometry. Strategy: A Draw the Lewis electron structure of the molecule or polyatomic ion. B Determine the electron group arrangement around the central atom that minimizes repulsions. C Assign an AX m E n designation; then identify the LP–LP, LP–BP, or BP–BP interactions and predict deviations in bond angles.
Draw the Lewis electron structure of the molecule or polyatomic ion. Determine the electron group arrangement around the central atom that minimizes repulsions. Assign an AX m E n designation; then identify the LP–LP, LP–BP, or BP–BP interactions and predict deviations from ideal bond angles. Describe the molecular geometry. The molar mass and melting point of beryllium chloride are 79.91 g/mol and 399 °C, respectively. The chemical bonding in Beryllium Chloride is studied by writing down its Lewis structure by following the Lewis approach. After lewis structure, there is a need of understanding its molecular geometry and hybridization of the central atom, Beryllium.
i3- lewis structure molecular geometry|I3
PH0 · Lewis Structure for I3
PH1 · I3 Lewis Structure, Molecular Geometry, Hybridization, Polarity,
PH2 · I3
PH3 · I3